Research
The value of clinical research.
SIR Foundation strongly encourages research initiatives that advance patient care. We support the development of trials, clinical registries and other research programs.
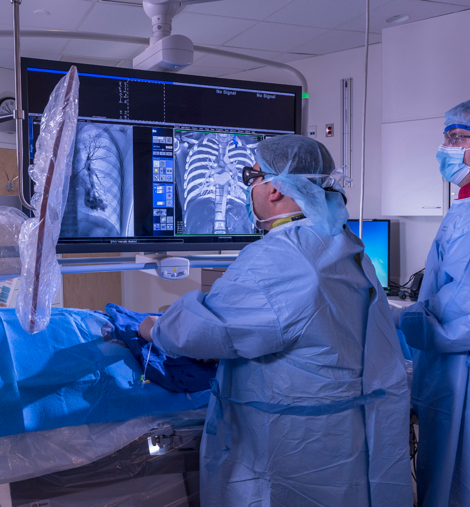
Clinical research to improve outcomes
SIR Foundation supports diverse research initiatives in IR. This can involve investigating a drug, device or procedure to evaluate health outcomes, improve patient care and medical knowledge.
Increase knowledge through clinical trials
SIR Foundation supports a variety of studies to advance IR. Involvement has included subject enrollment and raising awareness in the IR community.
Clinical registries. Collecting data, improving quality.
Clinical registries collect data on a specific population defined by a particular disease, condition or procedure. SIR Foundation encourages these endeavors as they support evidence-based research that can ultimately help improve quality and patient safety.
